- Définitions
- Éléments fondamentaux des services de prévention de l’AVC
-
1. Triage et évaluation diagnostique initiale de l’accident ischémique transitoire (AIT) et de l’AVC non invalidant
- Recommandations
- 1.0 AVC et accident ischémique transitoire
- 1.1 Risque ÉLEVÉ d’AVC récidivant (apparition de symptômes dans les dernières 48 heures)
- 1.2 Imagerie cérébrale et vasculaire
- 1.3 Analyses sanguines
- 1.4 Examens cardiaques
- 1.5 Évaluation fonctionnelle
- 1.6 Soins virtuels pour la prévention secondaire de l’AVC (Nouveau, 2020)
- 2. Prise en charge du mode de vie et des facteurs de risque
- 3. Pression artérielle et prévention de l’AVC
- 4. Prise en charge des lipides
- 5. Prise en charge du diabète et de l’AVC
- 6. Traitement antiplaquettaire des cas d’AVC ischémique et d’AIT
- 7. Traitement anticoagulant visant la fibrillation auriculaire
- 8. Prise en charge périopératoire du traitement anticoagulant et du traitement antiplaquettaire
- 9. Prise en charge de la maladie artérielle carotidienne extracrânienne et de l’athérosclérose intracrânienne
- 10. Problèmes cardiaques concomitants chez les personnes ayant subi un AVC
- 11. AVC ischémique associé au cancer
1. Triage et évaluation diagnostique initiale de l’accident ischémique transitoire (AIT) et de l’AVC non invalidant
Recommandations
- Ces recommandations (partie 1) concernent la prise en charge initiale des patients soupçonnés de présenter un AIT ou un AVC ischémique en phase aiguë et qui ne sont pas des candidats pour une thrombolyse ou une thrombectomie endovasculaire en phase aiguë. Pour les patients chez qui l’on soupçonne un AVC en phase aiguë qui justifie des évaluations en phase hyperaiguë afin de déterminer l’admissibilité à un traitement thrombolytique ou à une thrombectomie endovasculaire, veuillez consulter les recommandations relatives à la prise en charge de l’AVC en phase aiguë.
- Les personnes qui présentent des symptômes ou des signes d’un AVC en phase aiguë devraient immédiatement se rendre au service des urgences, idéalement en composant le 9-1-1 et en se faisant transporter par les services médicaux d’urgence pour amorcer rapidement le parcours préhospitalier.
- Dans les faits, certaines personnes présentant des signes ou des symptômes d’un AVC en phase aiguë seront plutôt vues en consultation externe (p. ex. par un médecin généraliste, un cabinet d’équipe de santé familiale, une clinique en milieu extrahospitalier ou un centre de soins urgents).
- Les personnes qui présentent des symptômes ou des signes d’un AVC en phase aiguë doivent faire l’objet d’une évaluation et d’un diagnostic rapides, ainsi que d’une évaluation rapide des risques relatifs à un AVC récidivant. Les patients qui ont reçu un diagnostic d’AIT ou d’AVC ischémique mineur qui ne sont pas des candidats au traitement thrombolytique en phase hyperaiguë par voie intraveineuse ni à la thrombectomie endovasculaire peuvent être considérés comme prioritaires pour une évaluation et une prise en charge de l’AVC en prévention secondaire.
- L’AVC ischémique est une affection hétérogène comportant de nombreux sous-types et pouvant être liée à diverses causes, des aspects qui ne peuvent être couverts par les présentes recommandations. Cette section se penche sur les études de diagnostic qui visent la reconnaissance des maladies les plus courantes (par exemple, l’athérosclérose ou la fibrillation auriculaire), mais aussi de certaines maladies plus rares nécessitant un traitement immédiat (par exemple, l’endocardite bactérienne).
1.0 AVC et accident ischémique transitoire
1.0 Les personnes ayant subi un AVC ou un AIT en phase aiguë qui sont vues en milieu ambulatoire (comme un milieu de soins primaires) ou à l’hôpital devraient faire l’objet d’une évaluation clinique par un professionnel de la santé spécialisé en soins de l’AVC afin de déterminer le risque d’AVC récidivant et d’amorcer les examens et les stratégies de prise en charge appropriés
1.1 Risque ÉLEVÉ d’AVC récidivant (apparition de symptômes dans les dernières 48 heures)
- Les personnes qui présentent des symptômes correspondant à un nouvel AVC en phase aiguë ou à un AIT (en particulier, des troubles focaux transitoires de la motricité ou de la parole ou des symptômes persistants d’un AVC) dans les 48 heures présentent le risque le plus élevé d’AVC récidivant. Elles doivent être aiguillées immédiatement vers un service des urgences (voir Facteurs cliniques 1.1.3) ayant la capacité de fournir des soins pour le traitement de l’AVC (notamment, une imagerie cérébrale sur place et, idéalement, un accès à des traitements d’AVC en phase aiguë) [niveau de preuve B].
- Il faut réaliser une imagerie cérébrale (TDM ou IRM) d’urgence, ainsi qu’une imagerie neurovasculaire concomitante (par exemple, une angiographie par TDM) dès que possible et avant la sortie du service des urgences [niveau de preuve B].
- Les patients vus 48 heures après l’apparition d’un AVC en phase aiguë ou d’un AIT doivent faire l’objet d’une évaluation clinique et d’examens complets dans les plus brefs délais par un professionnel de la santé ayant une expertise en matière d’AVC [niveau de preuve B]. Voir la section 2.2 pour en savoir plus sur les examens.
Section 1.1 Clinical Considerations:
- L’aiguillage vers un professionnel de la santé spécialisé dans les soins aux personnes ayant subi un AVC devrait être envisagé chez les patients chez qui on soupçonne une cause peu commune d’AVC, notamment les jeunes patients (âgés de moins de 45 ans) , en présence d’antécédents familiaux d’AVC à un jeune âge, en cas de possibilité de vascularite cérébrale ou d’une autre vasculopathie intracrânienne, ainsi qu’en cas de possibilité de thrombophilie héréditaire ou acquise.
- Les patients qui ont des symptômes d’une ischémie du territoire vertébrobasilaire présentent parfois également des symptômes fluctuants liés au tronc cérébral ou au cervelet (p. ex., diplopie, dysarthrie, dysphagie, vertige non positionnel, ataxie; rarement en tant que symptômes isolés) sur une période plus prolongée (c’est-à-dire, de plus de 48 heures) et peuvent donner la fausse impression d’un pseudo-AVC. Néanmoins, ces patients nécessitent eux aussi une évaluation, une imagerie neurovasculaire et une prise en charge d’urgence, puisque ces types d’AVC peuvent avoir une morbidité élevée. La consultation d’un professionnel de la santé spécialisé dans les soins aux personnes ayant subi un AVC est fortement encouragée.
- Contexte : Dans certaines régions, il existe des cliniques de soins rapides ou d’urgence des AIT. Ces cliniques disposent d’un accès rapide à des services de diagnostic et peuvent être considérées comme des choix pertinents en ce qui concerne l’aiguillage des patients ayant subi un AIT ou un AVC mineur.
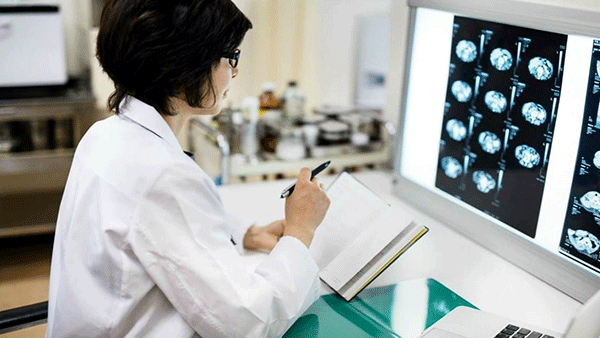
1.2 Imagerie cérébrale et vasculaire
- L’imagerie cérébrale (TDM ou IRM) et l’imagerie vasculaire non invasive (angiographie par TDM ou ARM de la crosse de l’aorte jusqu’au vertex) doivent être réalisées aussi vite que possible à la suite d’un AVC en phase aiguë ou d’un AIT [niveau de preuve B].
- L’angiographie par TDM de la tête et du cou (de la crosse de l’aorte jusqu’au vertex) qui peut être réalisée lors de la TDM initiale du cerveau est recommandée, car elle est idéale pour examiner la circulation extracrânienne et intracrânienne [niveau de preuve B]. Remarque : Certains établissements ne disposent peut-être pas d’un appareil d’angiographie par TDM immédiatement accessible. Aussi la planification et le type d’imagerie vasculaire doivent tenir compte des ressources disponibles et des protocoles locaux.
- L’imagerie neurovasculaire est recommandée pour repérer les patients présentant une sténose importante et symptomatique de l’artère carotide extracrânienne (c’est-à-dire une sténose de 50 à 99 %) et pour déclencher, le cas échéant, un aiguillage d’urgence en vue d’une éventuelle revascularisation carotidienne [niveau de preuve A].
- L’angiographie par TDM est l’examen d’imagerie vasculaire de première intention pour les personnes ayant subi un AVC ou un AIT. L’angiographie par résonance magnétique (ARM) et l’échographie carotidienne (pour l’imagerie vasculaire extracrânienne) sont des solutions de rechange raisonnables en tant qu’examens de première intention pour l’évaluation des carotides si l’angiographie par TDM n’est pas possible. Le choix doit se prendre en fonction des équipements disponibles et des caractéristiques du patient [niveau de preuve C].
Section 1.2 Facteurs cliniques:
- L’IRM cérébrale offre une meilleure sensibilité diagnostique que la TDM cérébrale en vue de la reconnaissance des petites lésions ischémiques chez les patients présentant cliniquement les symptômes d’un AIT ou d’un AVC mineur. Elle peut fournir des données supplémentaires pour guider le diagnostic, le pronostic et la prise de décision en ce qui concerne le traitement à réaliser. Les décisions concernant l’IRM doivent se faire en fonction de l’accès à l’IRM, de la disponibilité et du calendrier des rendez-vous. Pour un rendement diagnostique maximal, l’IRM devrait être effectuée dès que possible après l’événement symptomatique, idéalement dans les 7 jours suivant l’apparition des symptômes. L’IRM est particulièrement utile chez les patients à faible risque qui présentent des symptômes transitoires, chez qui la présence d’une ischémie pourrait justifier une modification de la prise en charge.
- Parmi les scénarios courants où une IRM cérébrale urgente peut être utile, on peut citer :
- TDM cérébrale normale malgré la persistance de symptômes au-delà de 24 heures (si l’IRM pondérée par diffusion est négative, une ischémie cérébrale est peu probable).
- Possibilité d’ischémie du tronc cérébral ou du cervelet (la TDM cérébrale n’a pas la sensibilité nécessaire à la détection d’un AVC dans la fosse postérieure à cause d’un artéfact osseux).
- Présence de symptômes focaux transitoires qui sont cliniquement atypiques de l’ischémie.
1.3 Analyses sanguines
- Les épreuves de laboratoire suivantes doivent être envisagées de façon systématique dans le cadre de l’évaluation initiale des patients présentant un AIT ou un AVC ischémique mineur :
- Hémogramme initial : formule sanguine complète, électrolytes, coagulation (TCA, RNI), fonction rénale (créatinine, débit de filtration glomérulaire estimé), glycémie aléatoire, ALT [niveau de preuve C]. Voir le tableau 1A pour la liste complète d’épreuves de laboratoire recommandées.
- Des épreuves de laboratoire supplémentaires peuvent être envisagées lors de la rencontre avec le patient ou en consultation externe, notamment un profil lipidique (à jeun et non à jeun) et un dépistage du diabète en mesurant le taux d’hémoglobine glyquée (HbA1c), la glycémie à jeun, ou l’hyperglycémie provoquée par voie orale à 75 g [niveau de preuve C]. Se reporter aux lignes directrices de Diabète Canada pour en savoir plus sur les épreuves de mesure de la glycémie.
- (NOUVEAU POUR 2020) : Si une artérite temporale est soupçonnée (par exemple, ischémie rétinienne ou céphalée), une analyse de la vitesse de sédimentation érythrocytaire et de la protéine C-réactive devrait être effectuée [niveau de preuve C].
- (NOUVEAU POUR 2020) : Les tests approfondis de thrombophilie visant les troubles d’hypercoagulation héréditaires ne sont pas recommandés pour l’examen habituel d’un patient atteint d’un AVC ischémique et devraient être limités à certaines situations (entre autres, la thrombose veineuse cérébrale inexpliquée, l’embolie paradoxale liée au FOP) [niveau de preuve C].
- Si l’on soupçonne un état d’hypercoagulabilité, il faut envisager de consulter un professionnel de la santé ayant une expertise en hématologie ou en thrombose [niveau de preuve C].
1.4 Examens cardiaques
1.4 A Détection de la fibrillation auriculaire
- Les patients chez qui on soupçonne un AVC ischémique ou un AIT devraient faire l’objet d’un ECG à 12 dérivations visant à permettre l’évaluation de la fibrillation auriculaire et le dépistage d’un éventuel infarctus du myocarde concomitant ou d’une cardiopathie structurelle (par exemple, une hypertrophie ventriculaire gauche) en tant que causes potentielles ou facteurs de risque d’un AVC [niveau de preuve B].
- Chez les patients examinés en raison d’un AVC ischémique d’origine embolique en phase aiguë ou d’un AIT, la surveillance ECG pendant 24 heures ou plus est recommandée en tant qu’élément de la prise en charge initiale de l’AVC, et ce dans le but de déceler une fibrillation auriculaire paroxystique chez les patients qui sont des candidats potentiels à une anticoagulothérapie [niveau de preuve A].
- Chez les patients examinés en raison d’un AVC ischémique d’origine embolique ou d’un AIT d’origine indéterminée dont la surveillance ECG initiale à court terme ne révèle pas de fibrillation auriculaire, mais chez qui on soupçonne un mécanisme cardioembolique, une surveillance ECG prolongée est recommandée pendant au moins deux semaines pour améliorer la détection d’une fibrillation auriculaire paroxystique chez certains patients âgés de 55 ans ou plus qui ne reçoivent pas encore d’anticoagulothérapie, mais qui sont des candidats potentiels au traitement [niveau de preuve A]. Reportez-vous à la section 7 du chapitre Prévention secondaire de l’AVC des Recommandations canadiennes pour les pratiques optimales de soins de l’AVC pour obtenir des conseils supplémentaires relatifs à la prise en charge des patients ayant subi un AVC et présentant une fibrillation auriculaire et pour connaître les dernières recommandations de la Société canadienne de cardiologie concernant la fibrillation auriculaire.
- (NOUVEAU POUR 2020) : Chez les patients âgés de plus de 65 ans qui ont subi un AVC ischémique ou un AIT, il est recommandé d’effectuer la palpation du pouls, l’auscultation cardiaque ou l’analyse de la bande de rythme d’électrocardiogramme afin de pouvoir dépister la fibrillation auriculaire non diagnostiquée [niveau de preuve B].
1.4 B Échocardiographie
- L’échocardiographie devrait être envisagée chez les patients ayant subi un AVC ischémique d’origine embolique ou un AIT de source indéterminée, ainsi que lorsqu’on soupçonne une étiologie cardioembolique ou une embolie paradoxale [niveau de preuve C]. L’échocardiographie systématique n’est pas nécessaire chez tous les patients victimes d’un AVC [niveau de preuve C].
- (NOUVEAU POUR 2020) : Chez les patients âgés de 60 ans ou moins qui sont examinés en raison d’un AVC ischémique d’origine embolique ou d’un AIT de source indéterminée, l’échocardiographie par contraste est recommandée pour la détection d’un éventuel FOP, si cela peut modifier la prise en charge du patient (notamment pour les patients qui seraient des candidats potentiels pour une fermeture du FOP ou une anticoagulothérapie si un FOP était détecté) [niveau de preuve B].
- L’échocardiographie transœsophagienne ou le doppler transcrânien par contraste (soluté physiologique agité) a une plus grande sensibilité que l’échocardiographie transthoracique pour ce qui est de la détection des shunts cardiaques et extracardiaques droite-gauche [niveau de preuve B].
1.5 Évaluation fonctionnelle
- Les patients ayant subi un AVC devraient être examinés pour vérifier l’absence de déficiences neurologiques et de limitations fonctionnelles (p. ex. évaluation cognitive, dépistage de la dépression, dépistage de la dysphagie, dépistage de la capacité à conduire, besoin de thérapie de réadaptation et assistance dans les activités de la vie quotidienne) [niveau de preuve B]. Se reporter au chapitre sur la réadaptation pour en savoir plus.
- Un aiguillage vers un spécialiste de la réadaptation approprié devrait être envisagé, aux fins d’évaluation approfondie et de prise en charge appropriée, lorsque les patients présentent des déficiences neurologiques et des limitations fonctionnelles [niveau de preuve B].
1.6 Soins virtuels pour la prévention secondaire de l’AVC (Nouveau, 2020)
- Les services de prévention secondaire de l’AVC doivent mettre en place des processus et des technologies permettant d’accroître et de garantir l’accès aux services par des modes de prestation de soins virtuels à l’intention des patients qui n’ont pas besoin de visites en personne, en particulier pour ceux qui vivent dans des zones rurales et éloignées sans accès local à des professionnels de santé ayant une expertise en matière d’AVC [niveau de preuve C]. Pour obtenir des renseignements et des conseils supplémentaires, se reporter à la trousse d’outils pour la mise en œuvre des soins de santé virtuels (2020) des Recommandations canadiennes pour les pratiques optimales de soins de l’AVC.
- Les cliniciens doivent suivre des critères établis et validés pour déterminer le meilleur mode pour chaque patient et pour chaque rencontre, en fonction du but et des objectifs de chaque visite [niveau de preuve C]. Se reporter au cadre décisionnel sur les soins virtuels de Cœur + AVC pour obtenir des recommandations et connaître les critères supplémentaires.
- La prise de décision concertée devrait également tenir compte des valeurs des patients, de leurs préférences et objectifs en matière de santé, ainsi que de la complexité médicale, des déterminants sociaux de la santé, et des besoins de santé propres à chaque patient [niveau de preuve C].
Section 1.6 Facteurs cliniques :
- Les établissements de consultation et les cliniciens doivent disposer de protocoles de triage et de critères d’admission locaux afin de s’assurer que les patients aiguillés vers leurs services seront examinés en temps utile, en particulier les patients à haut risque, conformément à la section 1.1 du présent chapitre.
- L’utilisation de soins virtuels visant la prévention de l’AVC devrait être accompagnée d’outils de décision permettant de distinguer les patients qui ont besoin d’un examen en personne de ceux qui peuvent raisonnablement être pris en charge dans le cadre des soins virtuels, ainsi qu’un mécanisme de planification des visites virtuelles qui favorise une approche d’équipe collaborative des soins administrés, s’il y a lieu et dans la mesure du possible. Se reporter au cadre décisionnel sur les soins virtuels de Cœur + AVC pour obtenir des recommandations et connaître les critères supplémentaires.
- Un plan d’urgence devrait être établi pour que les patients puissent être examinés en personne en temps utile si le besoin s’en fait sentir à la suite d’une rencontre de soins virtuels. Pour obtenir des renseignements et des conseils supplémentaires, se reporter à la trousse d’outils pour la mise en œuvre des soins de santé virtuels (2020) des Recommandations canadiennes pour les pratiques optimales de soins de l’AVC.
- Les évaluations virtuelles des patients visant la prévention secondaire des AVC doivent s’appuyer sur les thèmes définis dans la Liste de contrôle post-AVC et sur les éléments fondamentaux des soins de prévention de l’AVC. Pour obtenir des renseignements et des conseils supplémentaires, se reporter à la Liste de vérification après un AVC des Recommandations canadiennes pour les pratiques optimales de soins de l’AVC.
- Il convient de suivre des approches approuvées pour les examens neurologiques virtuels.
- Les contraintes liées à l’accès, à l’équité et à l’utilisation doivent être prises en compte, et des solutions de contournement doivent être mises en œuvre.
- Veillez à la mise en place de procédures relatives à la planification des rendez-vous pour les examens de suivi, pour l’aiguillage et pour les autres types de consultation à la suite d’une visite de soins virtuelle.
- Voyez à la documentation et à la communication appropriées pour les éventuels membres de l’équipe dont la participation aux soins se fait à distance.
- Encouragez les patients et leur famille à acquérir des dispositifs de surveillance à domicile de la pression artérielle, le cas échéant, et leur offrir des instructions et des ressources fiables portant sur l’utilisation de ces dispositifs. Pour les patients qui utilisent de tels dispositifs, il faut mettre en place des mécanismes de suivi et de gestion de la pression artérielle par les fournisseurs de soins primaires ou par les services de prévention de l’AVC.
- Pour assurer des épreuves diagnostiques en temps utile, envisagez l’utilisation de moniteurs cardiaques de longue durée, s’ils sont disponibles. Ces appareils peuvent être envoyés au domicile du patient et mis en place par lui-même, puis retournés par voie postale.
- Des mécanismes de collecte de données et d’amélioration de la qualité doivent être mis en place pour surveiller l’efficacité, l’efficience et la qualité des rencontres de soins virtuels.
L’objectif de la prise en charge en contexte extrahospitalier de l’AIT et de l’AVC ischémique non invalidant est une évaluation et une prise en charge rapides visant la réduction du risque d’une récidive potentiellement plus grave.
Les données montrent clairement que les AIT et les AVC mineurs sont des états instables qui constituent un avertissement quant au risque élevé d’AVC, d’un autre événement vasculaire ou de décès. Le risque d’AVC récidivant à la suite d’un AIT est de 4,7 % dans les 90 jours (Shahjouei et coll. 2020), et il va en décroissant. En effet, 3,8 % des AVC récidivants surviennent dans les deux premiers jours suivant l’apparition des premiers symptômes. Ces taux améliorés par rapport aux 20 % précédents témoignent de l’importance et des avantages d’une prise en charge intensive immédiate des patients ayant subi un AVC ou un AIT pour la prévention des AVC récidivants. Le risque d’AVC à sept jours peut atteindre 36 % chez les patients ayant subi un AIT qui présentent des facteurs de risque multiples. Il est prouvé que l’amorce en temps opportun de traitements en vue de la prévention secondaire et l’endartériectomie carotidienne peuvent réduire sensiblement le risque d’un AVC majeur à la suite d’un AIT ou d’un AVC ischémique non invalidant initial. Une étude du groupe TIARegistry.Org a révélé de nouveaux taux, qui étaient bien inférieurs à ce que révélaient les anciens groupes, et qui sont peut-être liés à la mise en place plus efficace et plus rapide des stratégies de prévention secondaire de l’AVC dans ce groupe, grâce aux consultations à accès rapide ciblant l’AIT (N Engl J Med 2016;374:1533-42). Ce type de baisse a été observé aussi au Canada (Kapoor et coll. 2020) en mettant l’accent sur le besoin de poursuivre la mise en œuvre de stratégies dynamiques de prévention secondaire pour éviter que ces taux n’augmentent à nouveau.
Les personnes ayant une expérience vécue, de même que leurs familles, insistent, elles aussi, sur l’importance d’un accès précoce à l’évaluation et au diagnostic pour prévenir les récidives. Elles soulignent l’importance des renseignements opportuns sur les signes et les symptômes de l’AVC et des explications claires sur le risque d’AVC récidivant. Des délais établis pour les personnes ayant différents niveaux de risque de récidive sont également importants. Le temps d’attente entre un premier AIT et les examens plus approfondis est parfois une période stressante, et ce fait devrait être pris en considération lors de la planification de la prise en charge. Elles ont également exprimé des inquiétudes quant aux préjugés signalés par certaines femmes, notamment en cas d’AIT ou de symptômes fluctuants.
-
L’éducation du public et des professionnels de la santé (généralistes, spécialistes et médecins en soins actifs) sur l’urgence de procéder à l’évaluation et à la prise en charge de l’AIT et de l’AVC ischémique non invalidant est d’une importance cruciale dans la réduction du risque de récidives potentiellement plus graves.
-
Il faut mettre en place des mécanismes pour offrir aux patients et aux familles qui en ont besoin des activités continues d’éducation et de soutien sur la prévention, la prise en charge de l’AVC, et les facteurs de risque associés.
-
Des séances de sensibilisation et de formation doivent être offertes aux professionnels de la santé qui travaillent dans des milieux de soins primaires, secondaires et tertiaires afin d’assurer une prise en charge en temps opportun des patients ayant subi un AIT ou un AVC ischémique mineur. Ces séances doivent également porter sur les liens entre le cœur et le cerveau et sur la nécessité d’aborder les soins de manière holistique, en tenant compte de tous les facteurs de risque vasculaire.
-
Les processus, protocoles et infrastructures nécessaires doivent être en place dans les services de santé communautaires et les établissements de soins actifs afin d’accéder rapidement aux épreuves diagnostiques et aux experts requis dans la prise en charge des patients avec AIT ou AVC mineur.
-
Des cliniques de prévention des AVC bien établies et accessibles ou des programmes plus généraux de prévention des maladies vasculaires doivent être accessibles dans toutes les communautés, aussi bien de la façon traditionnelle qu’à l’aide des technologies; des voies d’aiguillage et la promotion de programmes pour les professionnels de la santé doivent aussi être en place afin de favoriser un accès rapide. Ces ressources doivent figurer sur des listes mises à jour chaque année et être aisément accessibles pour les médecins de première ligne et les autres fournisseurs de soins de santé.
-
Les systèmes de soins de l’AVC doivent concevoir des modèles de services de soins virtuels afin d’améliorer l’accessibilité des services de prévention secondaire pour les patients des régions rurales et éloignées, ainsi que pour les patients qui ont des difficultés à se rendre en personne à leurs rendez-vous.
-
Nouveau, 2020 – Le coût et la durée des déplacements peuvent constituer un obstacle pour les habitants des régions rurales et éloignées qui souhaitent accéder à des services spécialisés éloignés. Il arrive souvent que les personnes refusent les demandes de consultation ou ne se présentent pas aux rendez-vous en raison du temps de déplacement, du coût et des mauvaises conditions météorologiques, surtout en hiver. Les soins virtuels transfrontaliers (interprovinciaux et provinciaux-territoriaux) doivent surmonter de nombreux obstacles réglementaires en ce qui a trait à la réglementation des professions en santé.
-
Des mesures de surveillance, d’évaluation et d’amélioration du programme concernant l’utilisation, l’observance et la qualité des programmes de prévention de l’AVC doivent être mises de l’avant pour garantir que les patients ont accès à des services efficaces. Il convient de réfléchir aux obstacles collectifs et individuels ainsi qu’aux éléments facilitants et aux éléments stimulants.
-
Nouveau, 2020 – Soins virtuels : Les gouvernements et les organisations doivent réfléchir aux moyens de surmonter les obstacles relatifs à l’accès et à l’utilisation des soins.
-
Afin d’améliorer l’accès aux tests pour les patients qui ont besoin d’une surveillance par moniteur Holter dans le cadre de l’évaluation de leur AVC, la livraison à domicile de moniteurs ECG ambulatoires peut être envisagée dans certaines régions.
- Proportion des patients ayant subi un AVC en phase aiguë ou un AIT dont la sortie du service des urgences ou d’un séjour à l’hôpital a eu lieu, mais qui sont réadmis pour n’importe quelle raison, et ce moins de 7 jours après leur sortie de l’établissement de soins actifs pour l’AVC en phase aiguë de référence (indicateur de qualité clé).
- Proportion des patients ayant subi un AIT ou un AVC non invalidant qui sont évalués par le service des urgences, qui obtiennent leur congé et qui, au moment de leur sortie, sont aiguillés vers un service organisé de prévention secondaire de l’AVC (indicateur de qualité clé).
- Délai entre la première visite pour des soins médicaux (soins primaires ou service des urgences) et l’évaluation par un spécialiste de l’AVC (en clinique ou dans un autre contexte de soins).
- Proportion des patients présentant un AIT et des symptômes moteurs et langagiers qui subissent une TDM cérébrale et une angiographie par TDM (ou un autre examen d’imagerie vasculaire) dans les 24 heures.
- Délai entre la première visite pour des soins médicaux et une imagerie cérébrale (TDM/IRM), une imagerie vasculaire (Doppler des artères cervicales, angiographie par TDM ou ARM) ou un électrocardiogramme.
- Indicateur de qualité clé à mettre au point : Proportion des patients ayant subi un AIT ou un AVC non invalidant qui sont exposés au risque LE PLUS ÉLEVÉ, qui sont examinés et pris en charge dans les 24 heures par le service des urgences ou aiguillés vers un service organisé de prévention secondaire de l’AVC (indicateur de qualité clé).
Notes sur la mesure des indicateurs
- L’accès aux données et leur qualité lors de la première rencontre et des dates et heures de l’aiguillage.
- Données de soins primaires issues de la facture établie par le médecin. Il faut se fier à la classification internationale des maladies (codes CIM) et non au diagnostic du médecin, qui est peut-être moins précis.
- Les mesures liées à d’autres recommandations visant la prévention sont également applicables à cette recommandation, mais ne sont pas répétées ici.
Renseignements destinés aux dispensateurs de soins de santé
- Recommandations canadiennes pour les pratiques optimales de soins de l’AVC (les Recommandations) – Prise en charge de l’AVC en phase aiguë, imagerie neurovasculaire
- Chapitre des Recommandations sur la prise en charge de l’AVC en phase aiguë – Outils de dépistage et d’évaluation de la gravité de l’AVC
- Trousse d’outils pour les soins virtuels des Recommandations
- Cœur + AVC – Liste de vérification après un AVC
- Cœur + AVC – Signes VITE de l’AVC
- Cœur + AVC – Lignes directrices 2020 en matière de RCR et de SUC :
- Société canadienne de cardiologie – 2020 SCC/SCR – Lignes directrices globales pour la prise en charge de la fibrillation auriculaire
- Guides cliniques de Thrombose Canada
- Lignes directrices de pratique clinique de Diabète Canada
- Index des recommandations de l’Association de la maladie d’Alzheimer
- Recommandations de la Cinquième conférence canadienne de consensus sur le diagnostic et le traitement de la démence
- American College of Chest Physicians (ACCP), thérapie antithrombotique pour la TEV – Lignes directrices et rapport d’un panel d’experts de CHEST
- Lignes directrices sur l’anticoagulation de l’American College of Chest Physicians (ACCP)
- Info AVC – Échelle neurologique canadienne
- Exemples d’examens neurologiques virtuels :
- Hussona, M.A., Maher, M., Chan, D., Micieli, J.A., Jain, J.D., Khosravani, H., Izenberg, A., Kassardjian, C.D., Mitchell, S.B. The Virtual Neurologic Exam: Instructional Videos and Guidance for the COVID-19 era. Canadian Journal of Neurological Sciences, 2020; 00, 1-6. doi:10.1017/cjn.2020.96; Vidéos d’appui
- American Academy of Neurology
- CorHealth COVID-19 Stroke Memo #5 – RECOMMENDATIONS FOR AN APPROACH TO RAMPING UP IN-PERSON SECONDARY STROKE PREVENTION CLINIC SERVICES IN ONTARIO
- CorHealth: Secondary Stroke Prevention Resources:
- Stroke Prevention Infographic for Primary Care Providers
- Stroke Prevention Clinic Patient Summary
- Depression, Obstructive Sleep Apnea and Cognitive Impairment – DOC Screening Tool
Informations destinées au patient
- Cœur + AVC – Signes de l’AVC
- Cœur + AVC – Signes VITE de l’AVC
- Cœur + AVC : Information sur l’AVC
- Cœur + AVC : Information sur la fibrillation auriculaire
- Cœur + AVC – Votre cheminement après un AVC
- Cœur + AVC – Liste de vérification après un AVC
- Cœur + AVC – Facteurs de risque associés aux maladies du cœur et aux AVC
- Cœur + AVC – Prendre en main son rétablissement : Aide-mémoire pour les soins de santé virtuels 2020 (infographie)
- Cœur + AVC – Lignes directrices canadiennes en réanimation et en premiers soins
- Cœur + AVC – Soutien en ligne par les pairs
- Partenariat canadien pour le rétablissement de l’AVC – Vidéos
- Info AVC
- Association canadienne pour la santé mentale – Retrouver son entrain
Triage and Initial Diagnostic Evaluation of Transient Ischemic Attack and Non-Disabling Stroke Evidence Table and Reference List
- Evidence Table and Reference List 1a: Triage and Initial Diagnostic Evaluation of Transient Ischemic Attack and Non-Disabling Stroke
- Evidence Table and Reference List 1b: Virtual Care
Patients who present with TIA or minor stroke are at increased risk of recurrent stroke, particularly within the first week following the initial event. A systematic review conducted by Giles & Rothwell (2007) pooled the results from 18 studies, consisting of 10,126 patients with TIA. The risk of stroke at days 2 and 7 was 3.1% 5.2%, respectively. Perry et al. (2014) examined stroke risk in 3,906 patients with TIAs admitted to 8 emergency departments over a 5-year period. In this cohort, 86 patients (2.2%) developed subsequent stroke within 7 days, and 132 (3.4%) at 90 days. Purroy et al. (2012) reported similar frequency of recurrent stroke among 1,137 patients admitted to 30 centers in Spain, presenting with TIA. Recurrent events occurred in 2.6% of patients within 7 days and 3.9% within 90 days. Following the first 30 days, the risk of recurrent stroke appears to decline. Mohan et al. (2011) included the results from 13 studies of patients recovering from first-ever stroke who were participants of hospital and community-based stroke registries. The cumulative risks of stroke recurrence were 3.1% at 30 days; 11.1% at one year; 26.4% at 5 years; and 39.2% at 10 years. Callaly et al. (2016) followed 567 participants of the North Dublin Population Stroke Study. The reported cumulative incidence of stroke recurrence was 5.4% at 90 days, 8.5% at one year and 10.8% at 2 years with a 2-year case fatality of 38.6%. These findings highlight the value of assessing patients who present with suspected stroke or TIA according to time since onset of symptoms.
Rapid clinical assessment by stroke specialists and subsequent investigations to differentiate TIA and minor stroke from other potential causes are essential to ensure that secondary prevention strategies can be implemented as soon as possible. Urgent TIA clinics provide such a model of care. The TIAregistry.org project is a prospective registry designed to follow patients presenting with TIA or minor stroke over a 5-year period. Patients were included if the event had occurred within the previous 7 days. The preliminary one-year results, which included 4,583 patients recruited from 61 sites in 21 countries from 1997-2003, indicated that 78.4% of patients were seen by a stroke specialist within 24 hours of the event (Amarenco et al. 2016). Most patients received key urgent investigations before discharge and appropriate treatments were initiated. For example, 5.0% of patients received a new diagnosis of atrial fibrillation, of which 66.8% received anticoagulant therapy before discharge. Carotid stenosis of ≥50% was found in 15.5% of patients, of which 26.9% underwent carotid revascularization before discharge. The one-year estimate of risk of the primary outcome, a composite of death from cardiovascular causes, nonfatal stroke and nonfatal acute coronary syndrome, was 6.2% (95% CI 5.5-7.0%). Estimates of the stroke rate at days 2, 7, 30, 90, and 365 were 1.5%, 2.1%, 2.8%, 3.7%, and 5.1%, respectively. These estimates were much lower than those compared with historical cohorts and were attributed to the widespread establishment of TIA clinics. Rothwell et al. (2007) reported that patients who had immediate access to a TIA clinic (EXPRESS) had a significantly reduced risk of recurrent stroke (2.1% vs.10.3%, p=0.0001), compared with an historical cohort who did not have immediate access to the same care. Patients with immediate access also received their prescriptions sooner (median of 1 vs. 20 days). Lavallée et al. (2007) reported the 90-day risk of stroke for all patients seen at their TIA-SOS clinic was lower than that predicted by their ABCD2 score (1.24% vs. 5.96%).
Atrial fibrillation (AF) is a common arrhythmia, which is associated with an increased risk of ischemic stroke. Following minor stroke or TIA, detecting AF in patients with no previous history is important, particularly in those with a cryptogenic stroke or embolic stroke of unknown source. Once identified, AF can be effectively managed, typically with a switch from an antiplatelet to an anticoagulant. However, AF is under-diagnosed because it is frequently paroxysmal and asymptomatic, and patients do not routinely undergo prolonged screening. AF can be detected using a variety of methods including a12-lead electrocardiogram (ECG), Holter monitoring, event recorders and implantable devices. Low levels of monitoring were highlighted in a study authored by Edwards et al. (2016). The records of 17,398 consecutive patients presenting with first-ever stroke or TIA with motor or speech deficits, without a known history of AF in sinus rhythm, were reviewed and the utilization of ambulatory ECG monitoring within the first 90 days of the event was assessed. A total of 5,318 patients (30.6%) received at least 24-hour Holter monitoring within 30 days of the index event. The numbers associated with more prolonged Holter monitoring were lower; 2,253 patients (12.9%) and 25 patients (0.1%) underwent 48-hr and >60-hr monitoring, respectively within 90 days. Monitoring with event loop recording was conducted in 139 patients (0.8%) within 90 days. A meta-analysis conducted by Sposato et al. (2015) examined the use of outpatient cardiac monitoring following minor stroke or TIA in 4 distinct phases. The results from the studies that initiated investigations during the second ambulatory period (phase 4), using mobile cardiac outpatient telemetry (n=5), external loop recording (n=7) or implantable loop recording devices (n=7), reported an estimated 16.9% (95% CI 13.0% -21.2%) of patients were diagnosed with AF.
Prolonged ECG monitoring using wearable or insertable devices has been shown to be effective for improving the detection of paroxysmal AF (numbers needed to screen range from 8-14), with longer monitoring durations associated with an increased probability of AF detection. A systematic review and meta-analysis (Tsivgoulis et al. 2019) included the results from 2 RCTs (FIND-AF and Crystal AF and 2 observational studies). The outcomes of persons who received prolonged cardiac monitoring (PCM) using implantable cardiac monitoring or ambulatory ECG monitoring, were compared with patients who received conventional (non-PCM) cardiac monitoring. Among persons who received PCM, AF was detected more frequently (RR=2.46; 95% CI, 1.61–3.76), the risk of recurrent stroke and recurrent stroke or TIA during follow-up was significantly lower (RR=0.45; 95% CI, 0.21–0.97 and RR=0.49; 95% CI, 0.30–0.81, respectively) and anticoagulation therapy was initiated more frequently (RR=2.07; 95% CI, 1.36–3.17). In the FIND-AF trial, Wachter et al. (2016) recruited 398 patients, >60 years admitted with acute ischemic stroke, within 7 days of symptom onset, in sinus rhythm at admission and without a history of AF. Patients were randomized to receive prolonged Holter ECG monitoring for 10 days, starting in the first week post stroke, and repeated at 3 and 6 months or standard care (an average of 73 hours of inpatient telemetry plus an average of 24 hours of Holter monitoring). At both 6 and 12 months, detection of AF was significantly higher in the prolonged monitoring group (13.5% vs. 4.5% and 13.5% vs. 6.1%, respectively). The associated numbers needed to screen were 11 and 13. There were no significant differences between groups in stroke recurrence (2.5 vs. 4.5%, p=0.28) or death (3.0 vs. 4.5%, p=0.45). A UK trial (Higgins et al. 2013) that randomized 100 patients with no history of AF and in sinus rhythm, reported that a strategy of 7-day ECG monitoring in the acute phase post-stroke was superior to standard care for the detection of paroxysmal AF (18% vs. 2%; p<0.05). Significantly more patients that received additional monitoring were started on anticoagulants.
Among persons with nonacute stroke, Gladstone et al. (2014), found 30-day ambulatory cardiac event monitor to be superior to repeat 24-hour Holter monitoring in identifying AF in 572 patients aged 52 to 96 years without known AF, who had sustained a cryptogenic ischemic stroke or TIA within the previous 6 months. Atrial fibrillation lasting ≥30 seconds was detected more frequently in persons using the cardiac event monitor (16.1% vs. 3.2%, absolute difference, 12.9%; 95% CI 8.0 to 17.6; p<0.001; number needed to screen= 8). The cardiac event monitor was also more likely to identify cases of AF lasting longer than ≥2.5 minutes (9.9% vs. 2.5%, absolute difference, 7.4%, 95% CI, 3.4 to 11.3; p<0.001). By 90 days, oral anticoagulant therapy had been prescribed for more patients in the intervention group (18.6% vs. 11.1%, p=0.01). Three-quarters of AF cases identified in the intervention group were detected within the first 2 weeks of monitoring.
The clinical and cost-effectiveness of prolonged ECG monitoring are likely greater for patients with estimated good life expectancy and quality of life, and for those with excessive atrial ectopy, enlarged or poorly contracting left atrium, or elevated natriuretic peptide levels. While prolonged post-stroke ECG monitoring improves AF detection, it should be noted that clinical trials have not been powered to determine the effect of prolonged ECG monitoring on the rate of recurrent stroke. Device-detected AF is often brief and subclinical and the minimum duration or burden of device-detected AF that warrants initiation of anticoagulant therapy remains uncertain; therefore, expert opinion varies widely.
It has been estimated that 5% of all people over the age of 65 years, in Canada, have evidence of vascular cognitive impairment (VCI). The reported prevalence tends to be higher in those individuals who have experienced a stroke, with up to 29% developing VCI over 5 years following stroke (Pendlebury et al. 2015). Therefore, patients should be screened at the time of presentation using validated instruments such as the Montreal Cognitive Assessment test (MoCA) or the Mini-Mental State Exam (MMSE).
Laboratory investigations and assessment of physiological variables as part of a patient’s initial evaluation provides important information for patient management. A small case control study found that maintenance of normal physiological variables within the first three days of stroke has a beneficial effect on outcomes post stroke (Langhorne et al. 2000). Blood biomarkers have been shown to correlate with cerebral lesion size and stroke severity (Kisialiou et al. 2012). Ferrari et al. (2010) found that hypertension, diabetes, possible etiology, acute infection and cardiac abnormalities were all independent predictors of deterioration following TIA or minor stroke and recommended immediate diagnostic testing for their identification. Together, these findings suggest a complete evaluation of patients presenting with suspected stroke or TIA is beneficial for predicting risk of recurrent stroke and guiding patient management.
When in-hospital or in-clinic visits are not possible, some prevention interventions can be provided through virtual means, such as the telephone or computer-mediated communication. Virtual care interventions have been shown to effective for cardiovascular risk factor reduction. Monthly phone calls with a health advisor resulted in significantly lower systolic and diastolic blood pressures, and was also associated with significant improvements in diet, physical activity, drug adherence, and satisfaction with access to care, compared with usual care (Salisbury et al. 2016). Mobile health interventions were associated with a significantly reduced HgbA1C compared with the control condition and significantly increased odds of smoking cessation at 6 months (Liu et al. 2017). Digital health interventions including telemedicine, web-based strategies, email, mobile applications, text messaging, and monitoring sensors significantly reduced the risk of cardiovascular events (RR=0.61, 95% CI, 0.46–0.80, p<0.001) (Widmer et al. 2015).